Bulk Mechanical Characterization of Nanoparticle Concrete-Equivalent Mortars
ABSTRACT
The infrastructure needs of the country are rising sharply as roads, bridges, and buildings made of concrete fail to meet standards. The American Society for Civil Engineers estimates that the federal government would have to invest $3.6 trillion to bring America’s infrastructure up to acceptable standards by 2020. A novel form of concrete, with greater strength and durability due to nanoparticle (NP) addition, is required to fulfill these needs. NPs have been added to concrete, enhancing the strength and durability characteristics of the material. The goal of this study was to test how mass and surface area of NPs added would affect ultimate concrete strength. The NPs SiO2, TiO2, and Halloysite were added to concrete equivalent mortar (C.E.M.) mixtures from 0.27% – 1% the mass of cement and tested for compressive strength after 14 days. C.E.M.’s with a lower mass of SiO2 and Halloysite added demonstrated significantly higher strength than their higher mass counterparts and were not significantly different from the TiO2 samples of the same surface area. These results indicate that keeping the mass of NPs less than 1% the mass of the binder may have significant impact in increasing concrete strength and that surface area of NPs added may be a better predictor of expected strength than mass added.
INTRODUCTION.
For several decades, materials have been manipulated on the atomic scale to produce novel effects in an array of fields such as medicine, defense, and engineering. There is perhaps no field today that has explored nanotechnology and its applications more robustly than materials science. Nanoparticles (NPs) such as SiO2, TiO2, and Halloysite have been added to protective polymers, lasers, dyes, and even concrete to reveal emergent properties and enhanced characteristics [1,2]. As the mechanical demands on the infrastructure of the world continue to rise, it is becoming increasingly important to develop building materials which are up to those rigorous standards. Concrete is a promising candidate to meet the infrastructure needs of the present and the future seeing as it is the most widely produced and ubiquitously employed manmade material in the world [1].
Concrete is a complex, heterogeneous, multi-phase material with applications in defense, industry, and infrastructure. Cement is mixed with water to initiate a hydration reaction which produces mainly calcium silicate hydrate (C-S-H), the binding agent, and calcium hydroxide, an unused byproduct [1]. When coarse and fine aggregates such as barite and sand are added to the cement paste, the resulting mixture is known as concrete [1]. Due to its remarkable compressive strength, durability, affordability, and accessibility, concrete is used to build bridges, roads, dams, and structural aspects of buildings, from the foundation to the topmost pillars. In high strength forms, it has also shown promise in nuclear waste storage applications, such as the one investigated in this study.
There has been a spate of research into the application of nanotechnology in concrete to solve the infrastructure problems the world faces today. Scientists have conducted studies demonstrating significant gains in compressive strength of concrete due to the addition of NPs in the mixture [1,2,3,4]. Other important work in the area has focused on the durability characteristics of NP concrete in response to environmental factors leading to sulfate induced degradation and water induced decalcification [3,4]. As this research progresses, studies are likely to concentrate on translating the effects observed in the literature into practical solutions. Currently researchers examine exactly how much of a certain type of nanoparticle is best for a concrete employed in a sulfate rich environment, or how best to exploit novel strength characteristics to build stronger, safer, and longer lasting nuclear waste storage facilities [5,6].
In this study, high strength nanoparticle concrete-equivalent mortars (C.E.M.’s) were examined to address the problem of safely storing nuclear waste. A typical mix design for concrete currently used in such an application was first taken and reconfigured to produce a C.E.M. in which all of the coarse aggregate (CA) was replaced with fine aggregate (FA) of an equal surface area to the CA removed. Then, mixes were prepared by adding the NPs TiO2, SiO2, and Halloysite by one percent the mass of cement in the mix. Additional mixes were prepared by using the data on specific surface area of the NPs gathered by the ethylene glycol monoethyl ether (EGME) method and replacing a one percent mass of TiO2 NPs with an equivalent surface area of SiO2 and Halloysite. Previous studies on the effects of NPs in concrete have been based on the addition of these particles by mass; however, this study was novel in that it sought to examine how both mass and surface area of NPs added affected the bulk mechanical properties of a nanoparticle reinforced C.E.M. [3,4,5,6]. It was expected that the total mass of NPs added would not directly correlate with compressive strength, and that as the total surface area of the NPs added increased, there would be a corresponding increase in compressive strength due to increased hydration reactivity to form the binding agent C-S-H.
MATERIALS AND METHODS.
Measurement of Specific Surface Area of Nanoparticles by EGME Adsorption.
The specific surface area of the nanoparticles used in the C.E.M. mixes was determined by the EGME method [7]. Approximately 1 gram of each of the NPs seen in Table 1 was placed in a tare and oven dried for four days at 120°C. The samples were then taken out and reweighed, whereupon 3 mL of EGME was added to each tare to make a slurry. The samples were then placed in a vacuum desiccator at a pressure of 14 torr with a sample of CaCl2 to aid evaporation of EGME. The samples were taken out of the vacuum the next day, reweighed, and placed back in the desiccator. When successive weightings of the samples were seen to differ by 0.001 grams or less, they were taken out. The following formula, as detailed in the EGME procedure, was used to determine the specific surface area of the nanoparticles [7]:
\[Specfic\ Surface\ Area=\frac{W_a}{2.86\times{10}^{-4}\ W_s}\tag{1}\]
Wa was the weight of the EGME retained by the samples, 2.86 × 10-4 represented the weight of EMGE required to form a monomolecular layer on a square meter of surface, and Ws was the weight of NPs added initially.
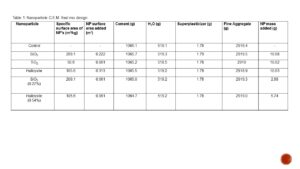
Table 1. The comprehensive strength of the respective mixes displayed almost no correlation when compared to the mass of NP’s added. The r² value is extremely low (0.01) indicating that almost none of the data is accounted for by the trend line.
Nanoparticle Reinforced Concrete-Equivalent Mortar Mix Design Process.
A typical concrete mix for a nuclear waste storage application consists of 297 kg of Portland Cement, 119 kg of H2O, 1320 kg of fine aggregate, and 1839 kg of coarse aggregate. In this study, a concrete equivalent mortar (C.E.M.) was used, in which the coarse aggregate was replaced with a mass of fine aggregate with an equivalent surface area to the typical mix. Equation 2 was used to find the specific surface area of the aggregates. By multiplying the respective specific surface areas of the aggregates by the mass used in a typical mix, the surface area of the mix was determined.
\[Specific\ Surface\ Area=\ \frac{Surface\ Area\ }{Volume\ \times S\ p\ e\ c\ i\ f\ i\ c\ Gravity}\tag{2}\]
\[Fine\ Barite\ Content=\ \frac{Total\ Mix\ Surface\ Area}{Fine\ Barite\ Specific\ Surface\ Area}\tag{3}\]
Equation 3 was used to find the amount of fine barite aggregate required in the mix to replace the surface area lost by the removal of the coarse aggregate. Since the fine aggregate used in this calculation was in a saturated surface dry state while the one used in the actual study was air dry, a quantity of water was added to the mix equivalent to the amount absorbed by the aggregate to maintain the water to cement ratio of 0.4. An equivalent mass of aggregate therefore had to be removed from the mix. The mass of aggregate required was determined using formulas relating moisture content to mass of air dry and saturated surface dry aggregate:
\[{MC}_A=\ \frac{M_A-\ M_D}{M_D}\tag{4}\]
\[{MC}_{SSD}=\ \frac{M_{SSD}-\ M_D}{M_D}\tag{5}\]
Where, MC was moisture content and M was mass, while the subscripts A and SSD represented air dry and saturated surface dry respectively. Rearranging the formula to solve for MA resulted in the following equation:
\[{M_A=\ {MC}_A+1\times\frac{M_{SSD}}{{MC}_{SSD}+1}}\tag{6}\]
Due to this correction 26 kg of aggregate was removed, and 26 kg of water was added to the mix to maintain a constant volume. Table 1 resulted from these calculations, showing the actual amounts of each component present in the mixes. In addition to cement, water, and fine aggregate, 1.78 grams of superplasticizer were added as per standards for a 2L mix [8]. Note that for NP samples of SiO2, TiO2, and Halloysite the mass added was at 1% of binder mass, and that for the 0.54% and 0.27% mass of binder Halloysite and SiO2 respectively the surface area added was equal to that of the TiO2 mix.
Casting and Curing.
The mixtures were cast into concrete beams and cured for 14 days before strength testing. For dispersion of NPs before casting, the NPs and the superplasticizer were sonicated for twenty minutes with part of the water for the mix. The ingredients for the C.E.M. were then poured into a bucket and mixed for three minutes. This was followed by a two minute rest period during which the researcher checked the mix for inconsistencies, and a final two minutes of mixing. Then, the C.E.M. was poured into two rectangular prism molds, tamped with a rod, and compacted with an air hammer to remove air bubbles. Finally, the molds were sealed with wrap overnight. The next day, the seals were removed and the C.E.M. was demolded and placed into a curing bucket with a moist paper towel. The bucket was sealed with a lid to trap the moisture.
Compressive Strength Analysis.
The compressive strength of the C.E.M.’s was tested to determine the impact of adding nanoparticles by mass and by surface area of binder. At 14 days after casting, the C.E.M. was taken out of the curing bucket and cut into five cubes with a diamond saw. The dimensions of these replicates was measured with a caliper, whereupon they were loaded, one by one, onto the Tinius-Olsen load frame. The load frame applied a force at a constant displacement rate of 1mm/s on each of the samples and generated a failure curve and an ultimate load for each. The strength of the samples was determined based on the ultimate load. Then, using a Student’s t-test at 95% confidence, the samples were analyzed with respect to one another and to the control.
RESULTS.
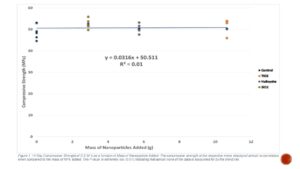
The 14 day compressive strength of the nanoparticle C.E.M.’s, when expressed as a function of mass of nanoparticles added to the mixes, showed almost no correlation (Figure 1). An extremely low r2 value (0.01) indicated that almost none of the data was explained by the trend line.
Figure 1. The compressive strength of the respecitive mixes displayed almost no correlation when compared to the mass of NP’s added. The r² value is extremely low (0.01) indicating that almost none of the data is accounted for by the trend line.
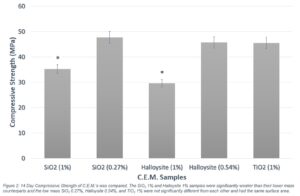
The mixes of SiO2 at 0.27% per mass binder and Halloysite at 0.54% per mass of binder demonstrated significantly greater strength than their respective 1% per mass mixes based on a student’s t-test to compare means at 95% confidence. The 0.27% per mass SiO2 mix displayed a 35.5% increase in strength over the 1% mix at 7 days and a 31.6% increase at 14 days (Figure 2). The lower mass Halloysite mix showed a 54.3% increase in strength over the 1% mix at 7 days and a 35.9 % increase at 14 days (Figure 2). Additionally, the TiO2 1% per mass, 0.27% SiO2 per mass, and 0.54% per mass binder Halloysite samples all added the same surface area to their respective mixes (Table 1 and Figure 2), but neither the 0.27% SiO2 nor 0.54% Halloysite samples demonstrated a significant change in strength when compared to the 1% TiO2 sample (Figure 2).
Figure 2. 14 day Compressive Strength of C.E.M’s was compared. The SiO2 1% and Halloysite 1% samples were significantly weaker than their lower mass counterparts and the low mass SiO2 0.27%, Halloysite 0.54%, and TiO2 1% were not significantly different from each other and had the same surface area.
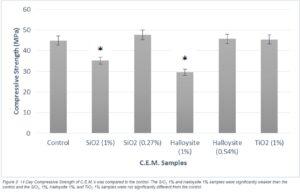
The TiO2 1% per mass, Halloysite 0.54% per mass, and SiO2 0.27% per mass binder mixes did not show a significant change in strength from the control 14 days. However, the Halloysite 1%, and SiO2 1% mixes both demonstrated significant decreases in strength compared to the control (Figure 3).
Figure 3. 14 day compressive strength of C.E.M’s was compared to the control. The SiO2 1% and Halloysite 1% samples were significantly weaker than the control and the SiO2 1%, Halloysite 1%, and TiO2 1% samples were not significantly different from the control.
DISCUSSION.
A number of studies focused on NPs in concrete have been based on the addition of NPs to the material incremented by mass of binder and have observed an increase in strength as the mass of NPs added to the mix increased [3,4,5]. According to Figure 1, the mass of NPs added seems to have no effect on the strength of the resulting C.E.M. The TiO2, Halloysite 0.54%, and SiO2 0.27% mixes all demonstrated marginally higher strength than the control, while the SiO2 1% and Halloysite 1% mixes had significantly lower strength than the control 14 days (Figure 3). Additionally, even though the 1% TiO2, 0.27% SiO2, and 0.54% Halloysite samples all involved differing amounts of NPs being added to the mix, they did not demonstrate a significant difference in strength (Figure 2). This indicates that addition of NPs in concrete by mass may not be the best predictor of ultimate strength. Furthermore, the 0.27% and 0.54% mixes of SiO2 and Halloysite respectively demonstrated significantly higher strength than the 1% mass mixes of both NPs at 14 days (Figure 2). Based on these results, it may be best to focus on adding NPs at less than 1% the mass of binder to optimize strength.
While the mass of NPs added was found to be a poor indicator of expected strength, the surface area of NPs present in a concrete mix was hypothesized to have a more predictable effect on the compressive strength of the material. In theory, NPs provide increased surface area for the hydration reaction to occur between cement and water, producing greater amounts of the binding C-S-H, and increasing compressive strength. The NPs which contributed the most surface area to the mix, Halloysite 1% and SiO2 1%, both had significantly lower compressive strength than the control at 14 days and the more surface area added by NPs the lower the strength (Figures 3 and 4). This result may have occurred due to agglomeration of NPs in the mix, leading to an effective surface area much lower than added. This result, coupled with the mass results, suggested that there was a fine balance between mass of NPs added to the mix, the total surface area they contributed, and the compressive strength. While a larger mass added does not seem to lead to gains in strength, an increase in surface area contributed by the NPs coupled with an observed decrease in strength suggested that agglomeration may be a problem which needs to be further addressed, and which is currently lacking in the literature.
CONCLUSION.
Studies in which the enhancement of concrete strength using NPs was examined are becoming increasingly important as the state of NP research has advanced and the infrastructure needs of the world have multiplied. This study examined how the mass and surface area of NPs added to C.E.M.’s affected the compressive strength of the materials. The findings indicate that adding NPs by more than 1% of the mass of cement in the mix may have been detrimental to the ultimate strength of the material, and that a higher surface area of NPs may have led to agglomeration and lower contribution to compressive strength in the concrete. However, while compressive strength seemed to have no correlation with mass of NPs added, surface area of NPs added did serve as a better predictor of expected strength.
Further work is needed to fully determine the ineffectiveness of mass as an indicator of expected strength. In future studies, NPs will be added at a lower mass range from 0-1% the mass of cement to determine the optimal mass for high strength NP concrete, a result which will be useful as research is translated into practical construction applications, especially in the development of nuclear waste storage facilities. If mass of NPs added is in fact not a significant factor contributing to concrete strength, then it would be more efficient and cost effective to build with a lower mass of NPs. The observed trend of lower compressive strength at increased NP surface area contribution also presents a viable conduit for future inquiry. The dispersion of NPs will be assessed using a scanning electron microscope to look for agglomerates. An abundant presence of these agglomerates will affirm that more effective dispersal is required in order to take advantage of the higher surface area contributed by the NPs and increase the compressive strength of the material. Further studies are also needed to assess the contribution of surface area of NPs to the compressive strength of the mix, specifically focusing on the question of effective NP dispersion and whether surface area of NPs added serves as a better indicator of expected strength than mass added.
ACKNOWLEDGMENTS.
I would like to thank Yonathan for being my steadfast and inspiring mentor throughout this project, Dr. Florence Sanchez for welcoming me into her lab and guiding me throughout the research process, and Dr. Lesa Brown for her poignant advice at both the inception and conclusion of this work.
REFERENCES.
- F. Sanchez, K Sobolev, Nanotechnology in Concrete – A Review. CBM. 11, 2060-2071 (2010).
- V. N. Rao, M. Rajasekhar, K. Vijayalakshmi, and M. Vamshykrishna, The future of civil engineering with the influence and impact of Nanotechnology on properties of materials. Pro. Mat. Sci. 10, 111–115 (2015).
- L. Brown and F. Sanchez, Influence of carbon nanofiber clustering on the chemo-mechanical behavior of cement pastes. Cem. Conc. Comp. 65, 101-109 (2016).
- Y. Zhou, M. Li, L. Sui and F. Xing, “Effect of sulfate attack on the stress–strain relationship of FRP-confined concrete,” CBM. 110, 235-250 (2016).
- I. Segura, Decalcification of cement mortars: Characterisation and modeling. Cem. Conc. Comp. 35, 136-150 (2013).
- L. H. J. Martin, A natural cement analogue study to understand the long-term behaviour of cements in nuclear waste repositories: Maqarin (Jordan). App. Geochem. 71, 20–34 (2016).
- Amy Cerato and Alan Lutenegger, Determination of Surface Area of Fine-Grained Soils by the Ethylene Glycol Monoethyl Ether (EGME) Method. Geotech. Test. Jour. 25, 1-7 (2002).
- L. Xiao, Z. Li, and X. Wei, Selection of superplasticizer in concrete mix design by measuring the early electrical resistivities of pastes. Cem. Conc. Comp. 29, 350–356 (2007).
Posted by Greta Clinton-Selin on Wednesday, June 7, 2017 in May 2017.
Tags: compressive strength, concrete, mass, Nanoparticles, nuclear waste storage, surface area